- The Avogadro Number
- Mole Avogadro's Number Worksheet
- Grams To Moles Using Avogadro's Numbers
- How To Use Avogadro's Number
- The Mole And Avogadro's Number Answer Key
Avogadro Number Calculations II
How Many Atoms or Molecules?
Problems 1 - 10
Use Avogadro’s number as a conversion factor to convert between moles and number of particles (atoms, molecules, ions, formula units.) Either: 6.022 x 10 23 particles 1 mole or 1 mole 6.022 x 1023 particles Problem: Convert 7.12 x 105 moles of SO 2 to molecules of SO 2. 7.12 x 105 moles x 6.022 x 10 23 molecules of SO 2 1 mole. To go back to the amount of our substance in moles when we have the number of molecules/atoms, all we need to do is divide that value by Avogadro’s Number. 4.5 x 10 23 molecules O 2 ÷ (6.022 x 10 23 molecules / mol) = 0.75 mol O 2.
- Quantities of substances such as what we weigh and use in the chemistry lab. AMUs, Grams, and Moles The value of Avogadro's number is actually chosen arbitrarily, based on the definition of the atomic mass unit, amu or u. By definition, a single carbon-12 atom weights 12 amu exactly. Therefore, one amu is one-twelfth the mass of a single carbon.
- You'll frequently convert moles of 1 thing in the reaction into moles of something else in the reaction using the coefficients in the balanced equation. In the bottom part, you'll use the molar.
Problem #1: How many atoms of chlorine are in 16.50 g of iron(III) chloride?
Solution:
1) Determine moles of FeCl3:
16.50 g / 162.204 g mol¯1 = 0.101723755 mol
2) Determine how many formula units of iron(III) chloride are in 0.1017 mol:
0.101723755 mol x 6.022 x 1023 = 6.1258 x 1022 formula units
3) Determine number of Cl atoms in 6.1258 x 1022 formula units of FeCl3:
6.1258 x 1022 x 3 = 1.838 x 1023 atoms (to 4 sig fig)
4) Set up using dimensional analysis:
| 1 mol | 6.022 x 1023 | 3 Cl atoms | ||||
| 16.50 g x | –––––––– | x | –––––––––– | x | ––––––––– | = 1.838 x 1023 chlorine atoms |
| 162.204 g | 1 mol | 1 FeCl3 |
Problem #2: How much does 100 million atoms of gold weigh?
Solution:

1) Determine moles of gold in 1.00 x 108 (we'll assume three sig figs in the 100 million):
1.00 x 108 atoms divided by 6.022 x 1023 atoms/mol = 1.660578 x 10¯16 mol
2) Determine grams in 1.66 x 10¯16 mol of gold:
1.660578 x 10¯16 mol times 196.97 g/mol = 3.27 x 10¯14 g (to three sig fig)
Problem #3: 18.0 g of (NH4)2CO3 is present. (a) How many oxygen atoms are present? (b) How many hydrogen atoms are present? (c) How many total atoms are present?
Solution:
1) Convert mass to moles:
18.0 g / 96.0852 g/mol = 0.18733374 mol
2) Determine how many formula units of ammonium carbonate are present:
(0.18733374 mol) (6.022 x 1023 mol¯1) = 1.128124 x 1023 formula units
3) Determine oxygen atoms present:
(1.128124 x 1023 formula units) (3 O atoms / formula unit) = 3.38 x 1023 O atoms
4) Determine hydrogen atoms present:
(1.128124 x 1023 formula units) (8 H atoms / formula unit) = 9.02 x 1023 H atoms
5) Determine total atoms present:
(1.128124 x 1023 formula units) (14 atoms / formula unit) = 1.58 x 1024 atomsFor the total, simply count up how many atoms are in the formula unit. In this case, there are 14 total atoms in one formula unit of (NH4)2CO3 (two N, eight H, one C and three O).
Problem #4: Suppose we knew that there were 8.170 x 1020 atoms of O in an unknown sample of KMnO4. How many milligrams would the unknown sample weigh?
Solution:
1) Determine how many formula units of KMnO4 there are:
8.170 x 1020 atoms divided by 4 atoms per formula unit2.0425 x 1020 formula units of KMnO4
2) Determine moles of KMnO4:
2.0425 x 1020 formula units divided by 6.022 x 1023 formula units/mol = 3.39173 x 10¯4 mol
3) Determine grams, then milligrams of KMnO4:
3.39173 x 10¯4 mol times 158.032 g/mol = 0.0536 g(0.0536 g) (1000 mg/g) = 53.6 mg
4) Dimensional analysis:
| 1 formula unit | 1 mol | 158.032 | 1000 mg | |||||
| 8.170 x 1020 atoms x | –––––––––––– | x | –––––––––– | x | ––––––– | x | ––––––– | = 53.6 mg |
| 4 atoms | 6.022 x 1023 | 1 mol | 1 g |
Problem #5: A solid sample of cesium sulfate contains 5.780 x 1023 cesium ions. How many grams of cesium sulfate must be present?
Solution:
1) Determine how many formula units of Cs2SO4 must be present:
5.780 x 1023 divided by 2 = 2.890 x 1023This is because there are 2 Cs atoms per one cesium sulfate formula unit.
2) Determine how many moles of Cs2SO4 are present:
2.890 x 1023 divided by 6.022 x 1023 mol¯1= 0.479907 mol
3) Determine grams of cesium sulfate:
0.479907 mol times 361.8735 g/mol = 173.7 g
Problem #6: A sample of C3H8 has 4.64 x 1024 H atoms. (a) How many carbon atoms does this sample contain? (b) What is the total mass of the sample?
Solution:
1) Convert from hydrogen atoms to C3H8 molecules:
4.64 x 1024 H atoms divided by 8 H atoms per C3H8 molecule = 5.80 x 1023 molecules of C3H8
2) C3H8 molecules to carbon atoms:
5.80 x 1023 molecules times 3 C atoms per molecule = 1.74 x 1024<---answer for (a)
3) Moles of C3H8:
5.80 x 1023 molecules divided by 6.022 x 1023 molecules / mole = 0.9631352 mol
4) Mass of C3H8:
0.9631352 mol times 44.0962 g/mol = 42.47 gto three sig figs, this is 42.5 g <---answer for (b)
Problem #7: If a sample of disulfur hexabromide contains 6.99 x 1023 atoms of bromine, what is the mass of the sample?
Solution:
1) We need the moles of bromine:
6.99 x 1023 atoms divided by 6.022 x 1023 atoms/mol = 1.160744 mol or Br atoms
2) Disulfur hexabromide's formula is S2Br6. That means there are 6 moles of Br for every one mole of S2Br6, therefore:
1.160744 mol / 6 = 0.193457 mol of S2Br6 present
3) The molar mass of S2Br6 is 543.554 g/mol:
543.554 g/mol times 0.193457 mol = 105.15 gto three sig figs, 105 g
4) Dimensional analysis:
| 1 mol | 1 mol | 543.554 g | ||||
| 6.99 x 1023 atoms x | –––––––––– | x | ––––––– | x | –––––––– | = 105 g |
| 6.022 x 1023 | 6 mol | 1 mol |
Problem #8: A sample of dinitrogen trioxide contains 0.250 moles of oxygen. How many molecules of the compound are present?
Solution:
1) Calculate moles of N2O3:
0.250 mol O times (1 mol N2O3 / 3 mol O) = 0.083333 mol N2O3
2) Calculate molecules of N2O3:
0.083333 mol N2O3 times (6.022 x 1023 molecules / mol) = 5.02 x 1022 molecules (to three sig figs)
Problem #9: Determine the number of oxygen atoms in 2.30 g of Al2(SO4)3.
Solution:
1) Here are the steps:
(a) 2.30 g divided by molar mass of Al2(SO4)3 = moles of Al2(SO4)3(b) moles of Al2(SO4)3 times 6.022 x 1023 = formula units of Al2(SO4)3
(c) formula units of Al2(SO4)3 times 12 = oxygen atoms in Al2(SO4)3
2) Let's build that up in dimensional analysis style:
| 1 mol | 6.022 x 1023 | 12 O atoms | ||||
| 2.30 g x | ––––––––– | x | –––––––––– | x | ––––––– | = 4.86 x 1022 oxygen atoms |
| 342.147 g | 1 mol | 1 | ||||
| ↑ step (a) ↑ | ↑ step (b) ↑ | ↑ step (c) ↑ |
Problem #10: How many potassium ions are there in 85.0 g of potassium sulfate (K2SO4)?
Solution:
| 1 mol | 6.022 x 1023 | 2 K atoms | ||||
| 85.0 g x | ––––––– | x | –––––––––– | x | ––––––––––– | = 5.87 x 1023 K atoms |
| 174.26 g | 1 mol | 1 formula unit of K2SO4 |
Problem #11: A solution of ammonia and water contains 2.10 x 1025 water molecules and 8.10 x 1024 ammonia molecules. How many total hydrogen atoms are in this solution?
Solution:
Ammonia's formula is NH3 and water's is H2O. Ammonia has three atoms of H per molecule and water has two atoms of H per molecule.
1) Ammonia's contribution:
8.10 x 1024 times 3 = 2.43 x 1025 H atoms
2) Water's contribution:
2.10 x 1025 times 2 = 4.20 x 1025 H atoms
3) Sum them up:
2.43 x 1025 + 4.20 x 1025 = 6.63 x 1025 H atoms
Problem #12: (a) How many water molecules are there in a 4.080 g sample of solid aluminum sulfate octadecahydrate? (b) How many oxygen atoms are there in the 4.080 g sample?
Solution:
1) The formula (and molar mass) of aluminum sulfate octadecahydrate is:
Al2(SO4)3⋅ 18H2O666.4134 g/mol
2) Convert 4.080 g of Al2(SO4)3⋅ 18H2O to moles:
4.080 g / 666.4134 g/mol = 0.006122326 mol
3) Determine formula units of Al2(SO4)3⋅ 18H2O in the 4.080 g:
(0.006122326 mol) (6.022 x 1023 formula units / mol) = 3.68686 x 1021 formula units
4) Every formula unit has 18 water molecules associated with it:
(3.68686 x 1021 formula units) (18 water molecules per formula unit) = 6.636 x 1022 water molecules (answer to a)
5) In 3.68686 x 1021 formula units of Al2(SO4)3⋅ 18H2O, there are a total of 30 oxygen atoms in each formula unit:
(3.68686 x 1021 formula units) (30 O atoms per formula unit) = 1.106 x 1023 O atoms (answer to b)
Problem #13: Determine how many atoms of hydrogen are in 20.0 grams of ammonium chloride.
Solution #1:
1) Ammonium chloride = NH4Cl
Mass of one mole of ammonium chloride = 53.4916 gMass of 4 moles of H = 4.0316 g
Fraction H in NH4Cl = 4.0316 / 53.4916 = 0.075368843
2) To determine the mass of hydrogen in a specific mass of ammonium chloride, multiply by the fraction of H in NH4Cl.
Mass of H = (20.00 g) (0.075368843) = 1.50737686 g
3) Determine moles of hydrogen:

1.50737686 g / 1.008 g/mol = 1.49541355 mol
4) Determine atoms of hydrogen:
(1.49541355 mol) (6.022 x 1023 mole¯1) = 9.00 x 1023 atoms
Solution #2:
1) Convert grams to moles:
20.0 g / 53.4916 g/mol = 0.3738905 mol
2) Convert moles to number of NH4Cl formula units:
(0.3738905 mol) (6.022 x 1023 formula units / mole) = 2.2515686 x 1023 formula units of NH4Cl
3) There are 4 atoms of hydrogen per formula unit:
(2.2515686 x 1023 formula units) (4 atoms / form. unit) = 9.01 x 1023 atoms (rounded to three sig figs)
Problem #14: How many atoms of mercury are present in 9.70 cubic centimeters of liquid mercury? The density of mercury is 13.55 g/cm3. Answer in units of atoms.
Solution:
1) Determine mass of mercury in 9.70 cm3:
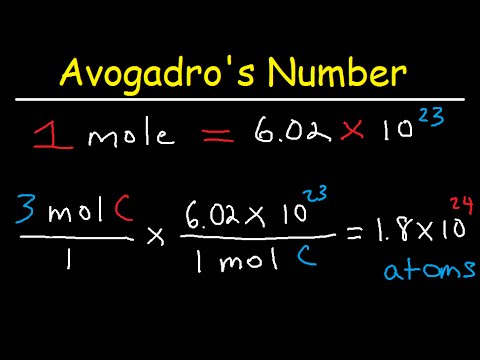
(9.70 cm3) (13.55 g/cm3) = 131.435 g
2) Determine moles of mercury in 131.435 g:
131.435 g / 200.59 g/mol = 0.655242 mol
3) Determine atoms in 0.655242 mol:
(0.655242 mol) (6.022 x 1023 atoms/mol) = 3.94 x 1023 atoms
Problem #15: What is the mass of CH4 molecules if they are made from 15.05 x 1023 atoms?
Solution:
1) In 'x' molecules of methane there are:
'x' atoms of C
'4x' atoms of H
2) From which follows this equation:
x + 4x = 15.05 x 1023x = 3.01 x 1023 atoms of C
3) Since there is 1 atom of C for every 1 molecule of CH4, we have:
3.01 x 1023 molecules of CH4
4) Calculate moles of CH4:
3.01 x 1023 molecules divided by 6.02 x 1023 molecules/mol = 0.500 mole of CH4
The Avogadro Number
5) Calculate mass:
0.500 mol times 16.0426 g/mol = 8.02 g (to three sig figs)
Problem #16: 3.00 L of hydrogen gas at SATP would contain how many atoms of hydrogen?
Solution:
1) SATP stands for Standard Ambient Temperature and Pressure and has the following values:
Temperature = 25.0 °CPressure = 100.0 kPa
Note that these values are different from STP. I found the values for SATP here. Look in the table, it's the sixth one down.
2) Use PV = nRT to determine moles of H2 present:
(100.0 kPa / 101.325 kPa/atm) (3.00 L) = (n) (0.08206 L atm / mol K) (298 K)n = 0.121076 mol
I converted kPa to atm because I have memorized the value for R I used. You can look up the value for R expressed in L kPa per mol K, if you wish.
3) Use Avogadro's Number to determine number of molecules:
(0.121076 mol) (6.022 x 1023 molecules/mol) = 7.2912 x 1022 molecules
4) Determine number of atoms:
(2 atoms/molecule) (7.2912 x 1022 molecules) = 1.46 x 1023 atoms
Bonus Problem: A sample of HNO3 contains twice as many atoms as there are atoms in 6.840 g of Al2(SO4)3. Calculate the mass of the HNO3 in the sample.
Solution:
1) We need to first determine the number of atoms in our sample of aluminum sulfate:
6.840 g / 342.147 g/mol = 0.0199914 molMole Avogadro's Number Worksheet
(0.0199914 mol) (6.022 x 1023 formula units/mol) = 1.203882 x 1022 formula units
(1.203882 x 22 formula units) (17 atoms / formula unit) = 2.0466 x 1023 atoms
2) The above calculation done in dimensional analysis style:
| 1 mole | 6.022 x 1023 form. units | 17 atoms | ||||
| 6.840 g x | ––––––– | x | –––––––––––––––––––– | x | ––––––––– | = 2.0466 x 1023 atoms |
| 342.147 g | 1 mol | 1 form. unit |
3) The sample of nitric acid will contain twice as many atoms:
(2.0466 x 1023 atoms) (2) = 4.0932 x 1023 atoms
4) Determine mass of HNO3, done in dimensional analysis style:
| 1 form. unit | 1 mol | 63.0119 g | ||||
| 4.0932 x 1023 atoms x | –––––––––– | x | –––––––––––––––––––– | x | ––––––– | = 8.566 g |
| 5 atoms | 6.022 x 1023 form. units | 1 mol |
Avogadro's number, also known as Avogadro's constant, is defined as the quantity of atoms in precisely 12 grams of 12C. The designation is a recognition of Amedeo Avogadro, who was the first to state that a gas' volume is proportional to how many atoms it has. This number is given as 6.02214179 x 1023 mol-1.
Grams To Moles Using Avogadro's Numbers
Amadeo Avogadro lived in the early 19th century and was an Italian savant known for his role in many different scientific disciplines. His most famous statement is known as Avogadro's Law, and is a hypothesis that states, 'Equal volumes of ideal or perfect gasses, at the same temperature and pressure, contain the same number of particles, or molecules.'
This is an intriguing hypothesis, because it says that quite different elements, such as nitrogen and hydrogen, still have the same number of molecules in the same volume of an ideal gas. While in real world settings this is not strictly true, it is statistically quite close, and so the ideal model still has a great deal of value.
The constant can be expressed as (p1)(V1)/(T1)(n1) = (p2)(V2)/(T2)(n2) = constant; where p is the pressure the gas is at, T is the temperature it is at, V is the volume of gas, and n is the number of molar units.
Part of Avogadro's genius, and while this number was named after him, is that he was able to see this fundamental relationship long before the experimental evidence was available to validate it. His innate understanding of the nature of ideal gasses was astounding, and it wasn't until decades later that experimental evidence finally supported his hypothesis.
In the 1860s, more than 50 years after Avogadro first made his hypothesis, the Austrian high school teacher Josef Loschmidt calculated how many molecules were in a single cubic centimeter of a gas under typical pressure and temperature. He determined this to be approximately 2.6X1019 molecules, a number now known as Loschmidt's Constant, and which has since been expanded to 2.68677725X1025 m-3.
Throughout the early years of the 20th century, a search was undertaken to discover the precise value of Avogadro's number. Molecules were still largely theoretical entities to many scientists until the early part of the 20th century, and so actually determining the value through experiment was not feasible. Once it became feasible, however, it was immediately apparent that the value was important, as it reflected on the fundamental nature of ideal gasses.
How To Use Avogadro's Number
The name 'Avogadro's number' was first used in a paper from 1909, by the scientist Jean Baptiste Jean Perrin, who later went on to win the Nobel Prize in Physics in 1926. He stated in the paper that, 'The invariable number N is a universal constant, which may be appropriately designated 'Avogadro's Constant.'
The Mole And Avogadro's Number Answer Key
For years leading up to the 1960s, there was some dispute as to the actual value of this number. Some factions used oxygen-16 to base their calculation on, while others used a naturally occurring isotope of oxygen, leading to slightly different values. In 1960, the constant was changed to be based on carbon-12, making the number much more regular.
